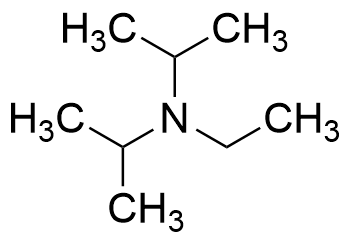
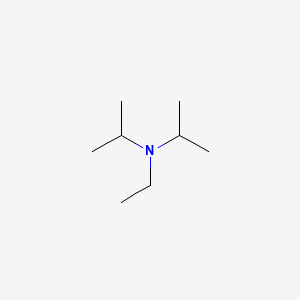
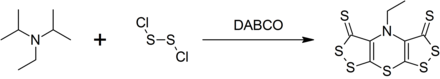Radical Reactions Induced by Visible Light in Dichloromethane Solutions of Hünig's Base: Synthetic Applications and Mechanistic Observations - Böhm - 2016 - Chemistry – A European Journal - Wiley Online Library
Synthesis of o-carboranyl analogues of rofecoxib 4a-c; (i) Hünigs base... | Download Scientific Diagram

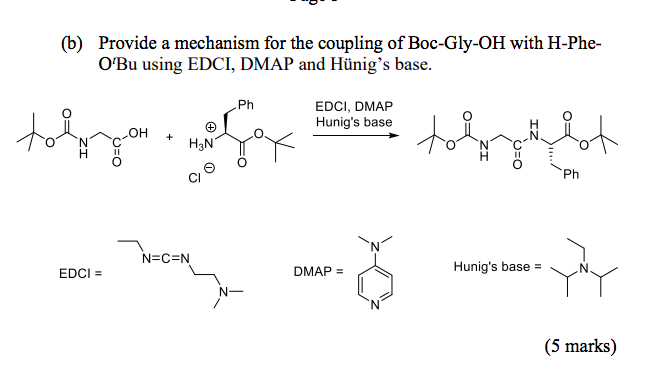
Ru-TsDPEN with Formic Acid/Hünig's Base for Asymmetric Transfer Hydrogenation, a Practical Synthesis of Optically Enriched N-Propyl Pantolactam | The Journal of Organic Chemistry
![Selective Syntheses of Bis[1,2]dithiolo[1,4]thiazines and Bis[1,2]dithiolopyrroles from Hünig's Base | The Journal of Organic Chemistry Selective Syntheses of Bis[1,2]dithiolo[1,4]thiazines and Bis[1,2]dithiolopyrroles from Hünig's Base | The Journal of Organic Chemistry](https://pubs.acs.org/cms/10.1021/jo971864e/asset/images/large/jo971864en00001.jpeg)
Selective Syntheses of Bis[1,2]dithiolo[1,4]thiazines and Bis[1,2]dithiolopyrroles from Hünig's Base | The Journal of Organic Chemistry

Ru-TsDPEN with Formic Acid/Hünig's Base for Asymmetric Transfer Hydrogenation, a Practical Synthesis of Optically Enriched N-Propyl Pantolactam | The Journal of Organic Chemistry
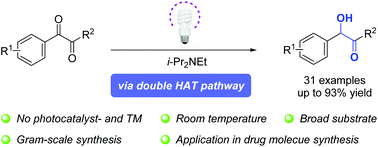
Visible-light enabled photochemical reduction of 1,2-dicarbonyl compounds by Hünig's base - Organic Chemistry Frontiers (RSC Publishing)

Radical Reactions Induced by Visible Light in Dichloromethane Solutions of Hünig's Base: Synthetic Applications and Mechanistic Observations - Böhm - 2016 - Chemistry – A European Journal - Wiley Online Library