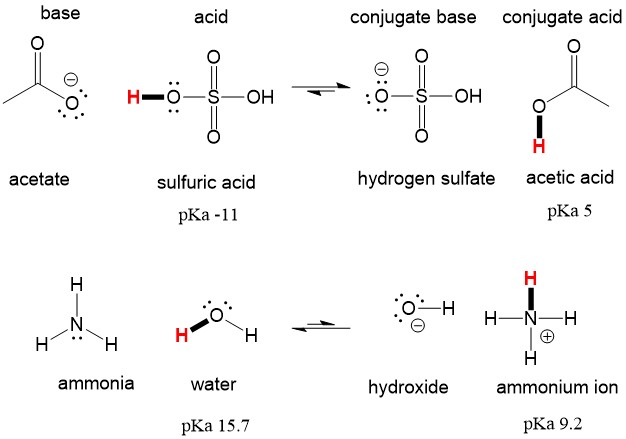
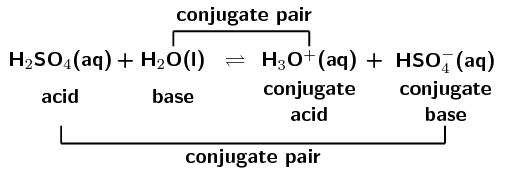
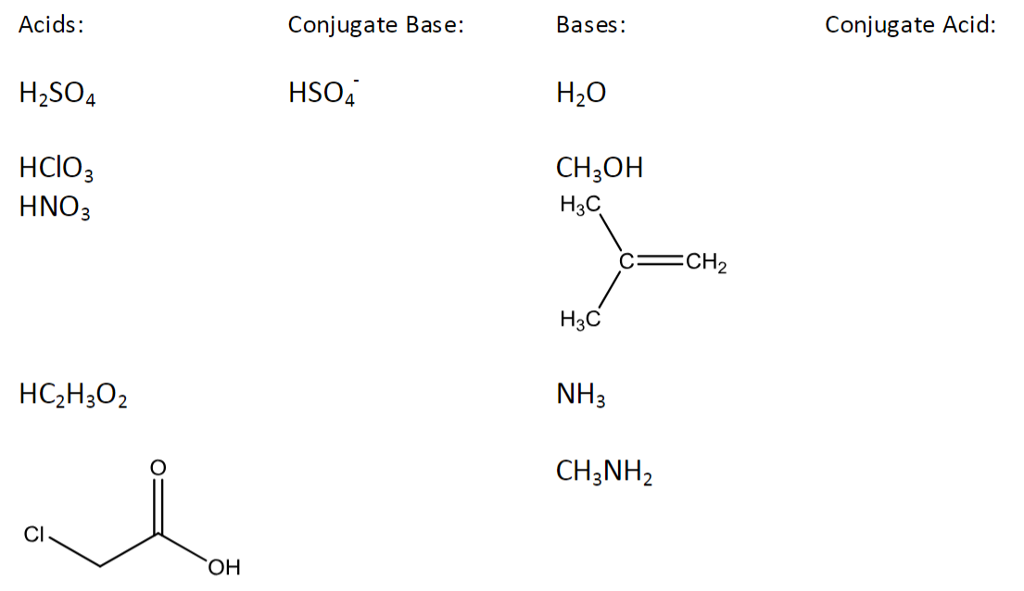
organic chemistry - Why Does A Brønsted–Lowry Acid Accept Proton from Stronger Acid? - Chemistry Stack Exchange
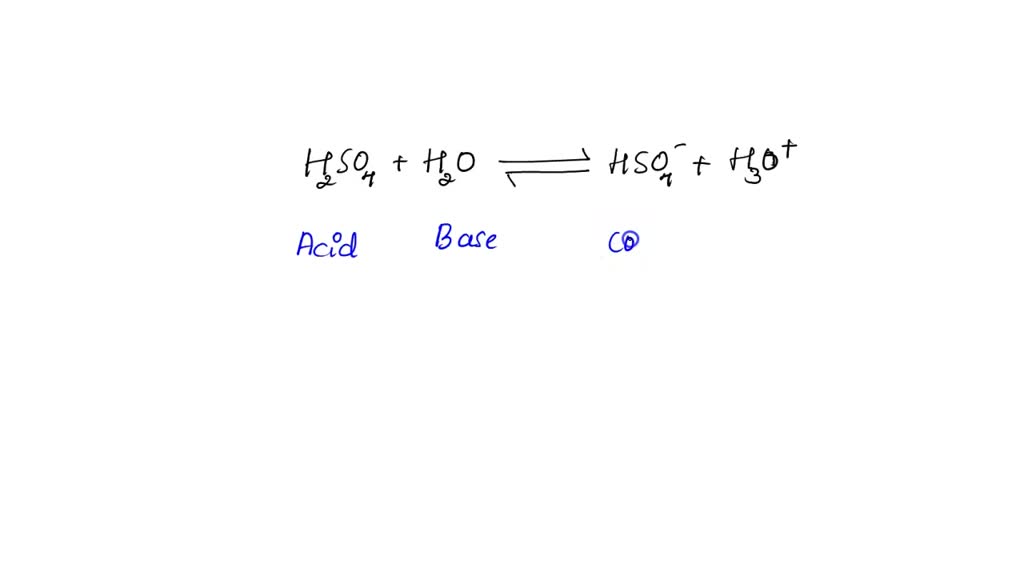
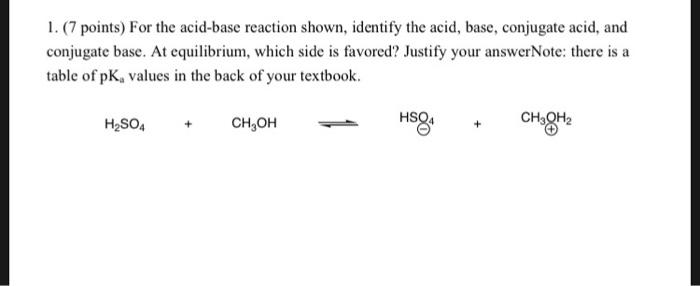
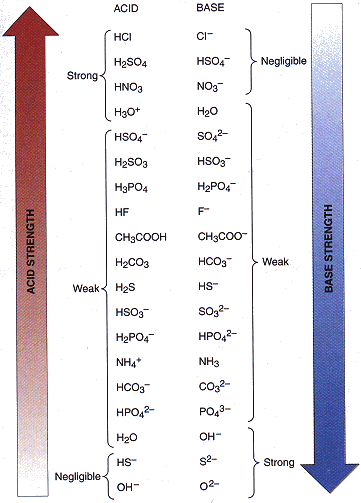
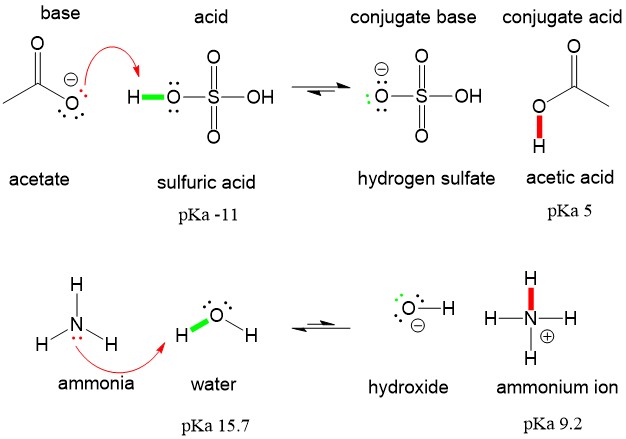
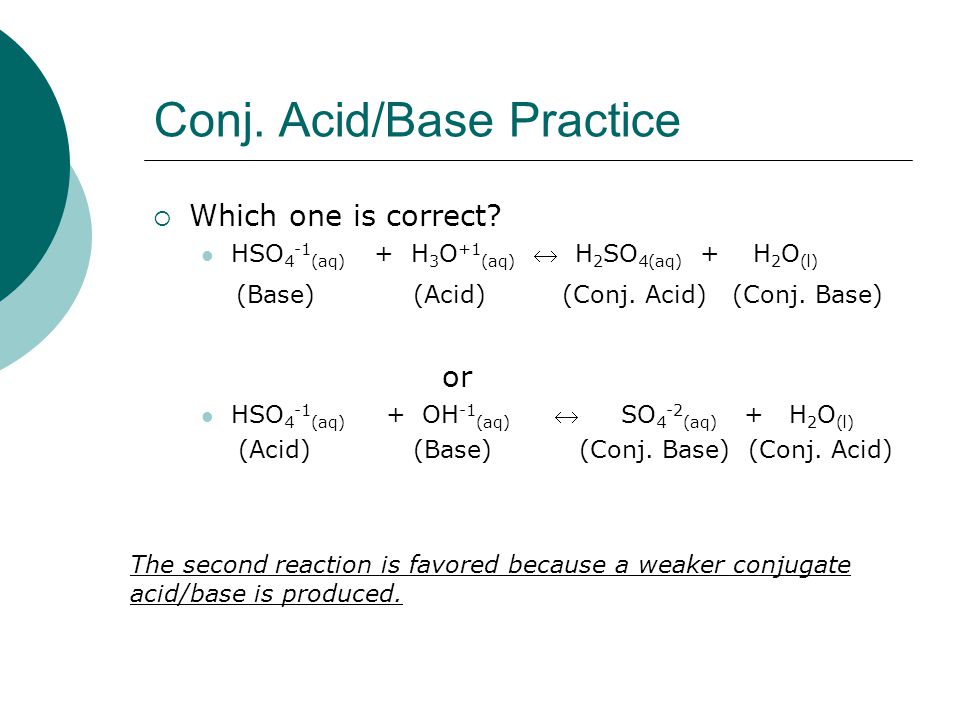
SOLVED: In the following equation, identify the Bronsted Lowry conjugate base H2SO4 + H2O <-> HSO4 - + H3O+ Group of answer choices H2SO4 HSO4- H2O H3O+